
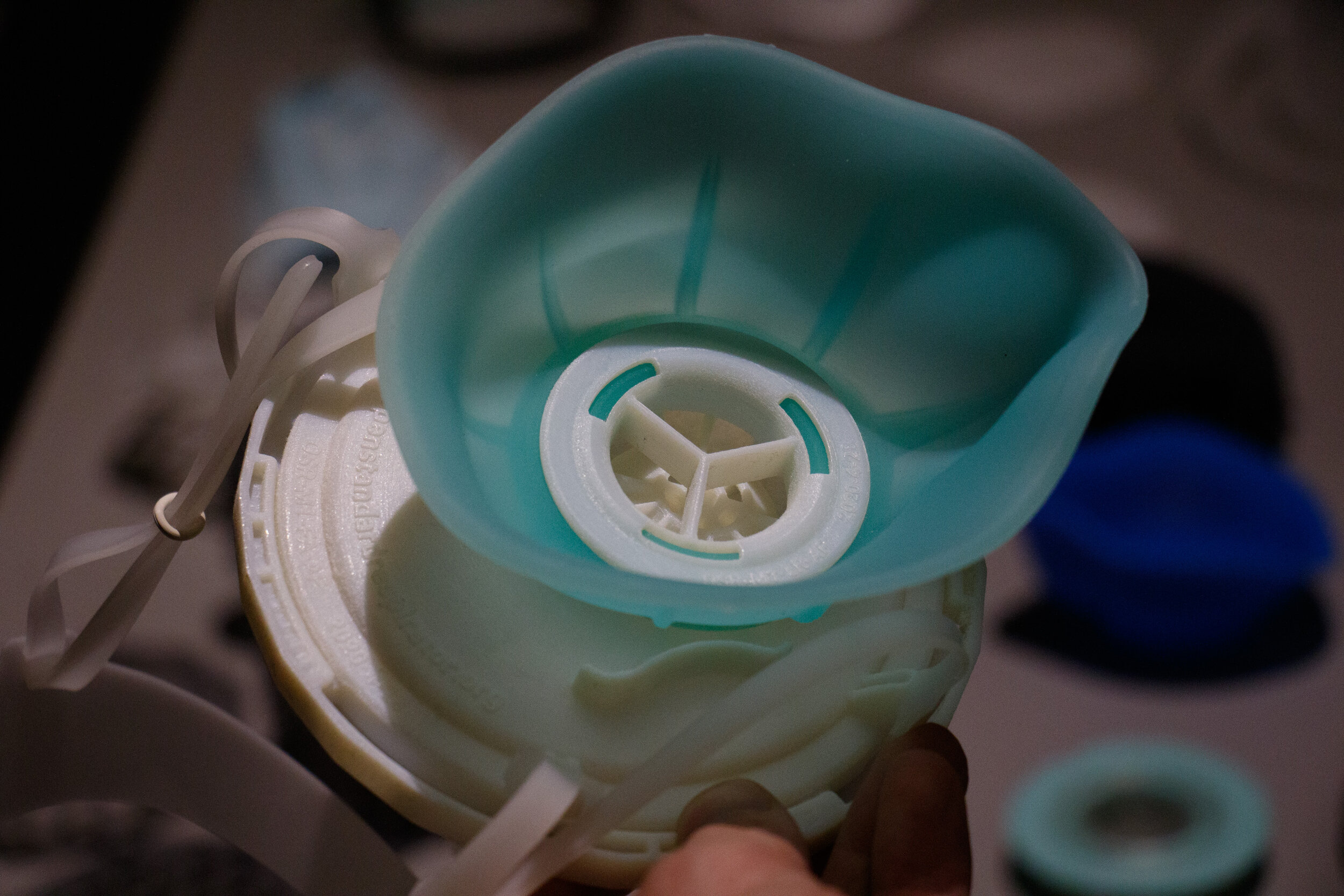
The OSR Model 1 is a reusable, elastomeric air-purifying face mask with bi-directional source-control filtering (no check-valve) and tight seal.
Now in production with our partner Open Standard Industries, Inc.
Pre-NIOSH Performance
Test data from three TSI8130a machines confirm filtration levels above 99% for 0.075um particles, while minimizing residual CO2 and breathing resistance — tested to 42 CFR part 84 specifications.
Find the Right Fit
The OSR-M1 modular design offers Small, Medium, Large sizes for individualized fitting. An Extra-Small facepiece for kids will be available soon.
Resilient PPE Supply Chain
The OSR’s replaceable filter disc uses 4x less material than a disposable mask, instantly expanding filter-media availability. The novel filter housing provides a scaffold for our certified filters, or in a pinch any available filtration media.
Sterilization Ready
The OSR-M1 elastomeric and rigid components are designed and tested to be compatible with standard decontamination techniques.
Cost Effective
The OSR-M1 pays for itself after 30 uses when compared to disposable PPE. Disposable PPE generates massive waste and is subject to highly volatile pricing and supply availability.
Modular Design
Designed with our future in mind, the components of the OSR-M1 can be easily replaced and upgraded as new designs are released with added functionality and features.
September 2020 Status Update:
The OSR-M1 has been undergoing pre-NIOSH particle filtration and blood-splash testing, in collaboration with AFFOA.org. Video copyright Andy Ryan.
Reusable, modular, low-cost N95 alternative
The OSR-M1 is a safe, user-friendly, reusable alternative to traditional N95 masks. The OSR-M1 is compatible with a wide range of available filter materials.
Bidirectional filtering
Form-fitting design for enhanced comfort
Compliant with CDC PPE guidelines

The Open Standard Respirator mission:
Develop a professional-grade protective mask
Make it accessible
Produce it locally and globally
Deploy it rapidly to those in need




Testing Status:
October 2020: Pre-NIOSH Testing is underway at two laboratories.
Exhalation Resistance - NIOSH TEB-APR-STP-0003
Inhalation Resistance - NIOSH TEB-APR-STP-0007
Residual CO2 - NIOSH RCT-APR-0064-508
Filter Efficiency - NIOSH TEB-APR-STP-0059
Blood Splash - ASTM F1862
Bacterial Filtration - ASTM F2101
The OSR Model 1 is targeted to be compliant with FDA EUA Guidance dated April 3, 2020 or later.








